Book Review: Lifespan
[epistemic status: non-expert review of a book on a highly technical subject, sorry. If you are involved in biochemistry or anti-aging, feel free to correct my mistakes]
David Sinclair - Harvard professor, celebrity biologist, and author of Lifespan - thinks solving aging will be easy. “Aging is going to be remarkably easy to tackle. Easier than cancer” are his exact words, which is maybe less encouraging than he thinks.
There are lots of ways that solving aging could be hard. What if humans worked like cars? To restore an old car, you need to fiddle with hundreds of little parts, individually fixing everything from engine parts to chipping paint. Fixing humans to such a standard would be way beyond current technology.
Or what if the DNA damage theory of aging was true? This says that as cells divide (or experience normal wear and tear) they don’t copy their DNA exactly correctly. As you grow older, more and more errors creep in, and your cells become worse and worse at their jobs. If this were true, there’s not much to do either: you’d have to correct the DNA in every cell in the body (using what template? even if you’d saved a copy of your DNA from childhood, how do you get it into all 30 trillion cells?) This is another nonstarter.
Sinclair’s own theory offers a simpler option. He starts with a puzzling observation: babies are very young [citation needed]. If a 70 year old man marries a 40 year old woman and has a baby, that baby will start off at zero years old, just like everyone else. Even more interesting, if you clone a 70 year old man, the clone start at zero years old.
(there were originally some rumors that cloned animals aged faster, but those haven’t been borne out)
This challenges the DNA theory of aging. A 70 year old’s skin cells have undergone seventy years of DNA damage, and sure enough the 70-year-old has weak, wrinkled skin. But if you transfer the skin cell DNA to an egg, inseminate the egg, and turn it into a baby, that baby is just as young as all the other babies. So DNA damage can’t be the whole story.
What could be an almost insurmountable problem for cells in a mature animal, but trivially vanishes when you clone a cell into an embryo?
Sinclair’s answer is epigenetics. Remember, all cells have the same DNA. The reason kidney cells are different from lung cells is because they have epigenetic markers on the kidney genes saying “turn these on” and on the lung genes say “turn these off”. Part of the cloning process involves telling the cell to be an egg cell. After that, it undergoes the normal embryogenesis process where embryonic stem cells differentiate into kidney cells, lung cells, and the rest.
So Sinclair thinks aging is epigenetic damage. As time goes on, cells lose or garble the epigenetic markers telling them what cells to be. Kidney cells go from definitely-kidney-cells to mostly kidney cells but also a little lung cell and maybe some heart cell in there too. It’s hard to run a kidney off of cells that aren’t entirely sure whether they’re supposed to be kidney cells or something else, and so your kidneys (and all your other organs) break down as you age. He doesn’t come out and say this is literally 100% of aging. But everyone else thinks aging is probably a combination of many complicated processes, and I think Sinclair thinks it’s mostly epigenetic damage and then a few other odds and ends that matter much less.
Epigenetic damage could potentially still be unfixable: how do you convince the thousands of different intermixed cell types in the body to all be the right type again? But Sinclair thinks the body already has a mechanism for doing this: epigenetic repair proteins called sirtuins. I’m a bit confused about where sirtuins are getting their information from: is there a backup copy of epigenetics that they read to figure out what’s wrong and needs repair? I get the impression from one or two cryptic statements that Sinclair thinks maybe yes (see the discussion of “the observer” on page 171). But for some reason, the system works well enough to keep you alive for the normal human lifespan (and no better).
If you want to live longer, can you just add more sirtuins? These people say they gave mice a gene that caused them to overproduce sirtuins, and the mice lived 30% longer. Other people have tried the same experiment in worms, fruit flies, etc, with controversial but generally positive results.
What if you’re not a mouse, and you live in one of the 100% of countries that have banned random irresponsible genetic engineering on humans? Sinclair thinks there’s still hope. Sirtuin activity seems to be regulated by a protein called mTOR (motto: “The Protein That Regulates Everything”, see discussion of its role in depression here, obesity here, cancer here, etc). In times of plenty, mTOR switches on, causing cells to divide and grow. In times of deprivation, mTOR switches off, causing cells to “hunker down” and go into power-saver mode. Apparently part of power-saver mode is damage control, so this turns on the sirtuins and makes them do more epigenetic repair, keeping you young. All you have to do to stay younger longer is keep mTOR from activating.
| [](https://substackcdn.com/image/fetch/f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fbucketeer-e05bbc84-baa3-437e-9518-adb32be77984.s3.amazonaws.com%2Fpublic%2Fimages%2F618e0176-4bc9-4de1-ac0b-6159d36225cc_735x441.jpeg)The main thing I remember from my biochemistry classes is that mTOR is a big oval with the word “mTOR” on it. |
mTOR turns off in times of deprivation, so you can keep it off by depriving yourself. That’s why the gold standard of anti-aging interventions is calorie restriction: eat less food. This isn’t just the “stay thin if you don’t want to die of a heart attack” thing, this is where you eat an absurdly low amount of food, practically starving yourself, as a desperate strategy to turn off this one protein. This kind of extreme calorie restriction extends lifespan about 50% in lemurs, and would probably work for humans too. Sinclair brings up the story of a weird Venetian merchant who took some kind of vow of temperance and ate famously little each day: he lived to 100, which is pretty good for the 1500s. And:
In more recent times, Professor Alexandre Gueniot, the president of the Paris Medical Academy just after the turn of the twentieth century, was famed for living on a restricted diet. It is said that his contemporaries mocked him - for there was no science at the time to back his suspicion that hunger would lead to good health, just his gut hunch - but he outlived them, one and all. He finally succumbed at the age of 102.
These stories are definitely cherry-picked, but we don’t have great studies: calorie restriction hasn’t been around the hundred years it would take to see results in humans. A few very committed biohackers having been trying it for a few decades now, so I guess we’ll know if it works by the mid-21st century.
What if you’re not a mouse, can’t get genetically engineered, and don’t want to starve yourself for your entire life. Then what? Some people think that you can simulation “deprivation” well enough to fool mTOR with intermittent fasting. Sinclair suggests maybe skipping breakfast and having a late lunch every day, or fasting entirely a few days a week, or a few weeks a year, or - well, nobody really knows how or whether intermittent fasting works, let alone the best way to do it. But it’s an option.
Or you could exercise, or go to a sauna, or go out when it’s really cold. Sinclair thinks all of these promote health by stressing the body and convincing it that it’s a bad time and it needs to go into power-saving mode, which activates sirtuins and repairs epigenetic damage.
Suppose you’re not a mouse, can’t get genetically engineered, and you have a normal aversion to diet and exercise. Is there a pill you can take? Yes! A suitably foreboding one, even! Rapamycin gets its name from Rapa Nui aka Easter Island, where it was discovered in a fungus living beneath one of the giant stone heads. It’s a strong inhibitor of mTOR (in fact, the “TOR” in mTOR’s name stands for “Target Of Rapamycin”). Mice on rapamycin live about 10% longer than usual. Can you take rapamycin? Probably a bad idea, it’s a potent immunosuppressant. Organ recipients take it sometime to quiet their immune system down to the point where it stops rejecting the transplant, but it’s not a lot of fun.
But there are two other pills that might work. One is resveratrol, a chemical found in red wine (though not in high enough doses to be meaningful for sirtuin activation). Resveratrol definitely activates sirtuins in test tubes, and seems to be good for lab animals: some of them live longer on it, others at least seem healthier. But the lab animal studies were never 100% conclusive, and arguably humans absorb it too poorly to be able to get an effective dose (I’m confused about some details here, like whether animals absorb it better, or whether IV formulations would work). There was a big mess surrounding claims by resveratrol supplement companies, whether their products might have worked or whether they couldn’t possibly have. David Sinclair was caught in the middle and got accused of being a Big Resveratrol shill, and scientific opinion seems to have settled as most against it. I think some people are now experimenting with pterostilbene, a more bioavailable resveratrol relative.
The other pill is nicotinamide riboside aka NR (and its close cousin nicotanimide mononucleotide aka NMN). The reactions catalyzed by sirtuins involve nicotinamides, and the more nicotinamides you have, the more effective sirtuins are. NR and NMN are cheap, simple chemicals you can buy at any supplement store for $20, and Sinclair is pretty convinced they’re a fountain of youth. He says that when his own father started becoming decrepit, he convinced him to take NMN, and over the space of a few months he started becoming energetic and spry again, and is now traveling the world despite being well into his 70s.
 Sinclair himself takes well above what other people would consider the maximum dose every day, and apparently looks like this at age 50.
Sinclair himself takes well above what other people would consider the maximum dose every day, and apparently looks like this at age 50.
Can sirtuins make us immortal? All of Sinclair’s examples involve slowing aging by 10 - 20%. I don’t quite understand why - if aging is just epigenetic damage, and epigenetic damage can be repaired, can’t you just increase the repair rate until it’s faster than the damage rate, then live forever? If Lifespan gave an explanation for this, I missed it.
That doesn’t mean we can’t be immortal, though. Sinclair’s lab has another research program, focusing on stem cells. These produce new, epigenetically healthy cells wherever they’re placed. And we now know that with a couple of chemicals called Yamanaka factors, you can make adult cells become stem cells again. Sinclair’s idea is to genetically engineer triggerable Yamanaka factors into the cells of human adults. Then, when you’re starting to feel old, you trigger the factors, some of your cells revert to stem cells, and they replace your old decaying cells with epigenetically healthy ones. Every biologist I mention this to has the same objection, which is “won’t that make you have every kind of cancer at once?”, and, in their defense, the first hundred times Sinclair tried this his mice got some pretty crazy cancers. But he swears they have solved this problem and the mice are doing fine now. Some of them are living about 40% longer than normal, which I notice still isn’t immortal but seems like a step in the right direction.
II.
People who are not David Sinclair generally don’t expect conquering aging to be this easy.
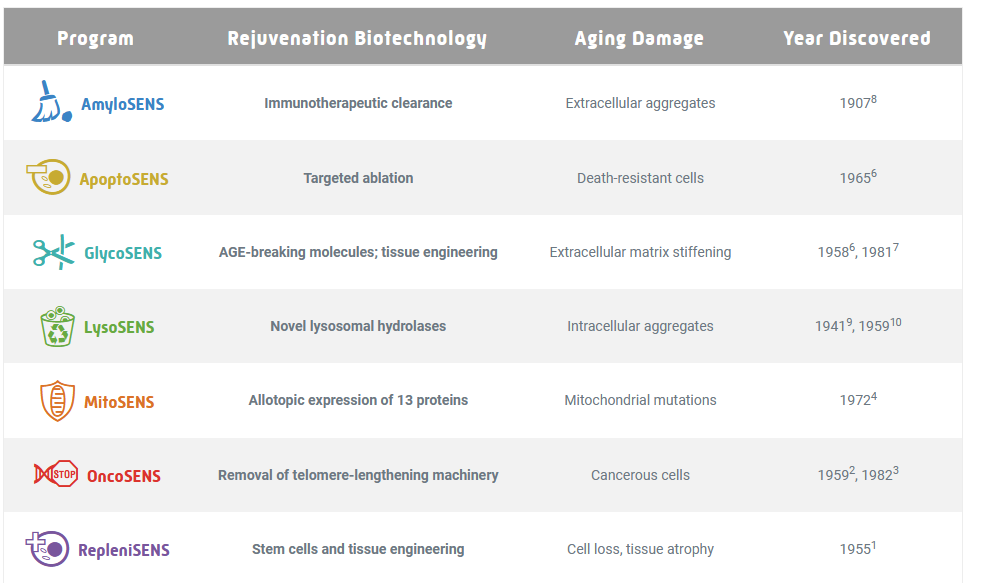
The anti-aging SENS Foundation has a list of seven different programs to address what they consider to be seven different causes of age-related damage. This seems more like the “humans are like cars” scenario where you have to fix every part individually and it’s really hard.
People who are not David Sinclair don’t think that nicotinamides are a miracle drug. A well-regarded research center ran a big study on nicotinamides in mice and found that they lived no longer than usual, although they did seem to be healthier in various ways. This might be a good time to mention that it’s really hard to run reliable aging studies in mice, because mice have a lot of natural lifespan variability, people don’t always use enough mice to constitute a good sample size, and you have to keep them around for years before you learn anything.
And people who are not David Sinclair are less enthusiastic about sirtuins, mTOR, and calorie restriction. Algernon’s Law says there shouldn’t be easy gains in biology. Your body is the product of millions of years of evolution - it would be weird if some drug could make you stronger, faster, and smarter. Why didn’t the body just evolve to secrete that drug itself? Or more to the point, since most drugs act by flipping biological “switches”, why does your body have a switch set to the “be weak, slow, and dumb” position? There are ways to answer this question, and drugs that do lots of great things. But any biohacking proposal does need to overcome this objection. So: why doesn’t the body just have more sirtuins? Why do sirtuins only repair epigenetic damage when mTOR is in the off position?
Also, everyone agrees that turning mTOR off increases lifespan. It increases lifespan by putting cells into power-saver mode so they do less. But if this mode is so great, why aren’t we in it all the time? The conventional answer is that in power-saver mode, you’re weaker, have less energy, and your wounds don’t really heal - which common-sensically matches what you’d expect of a power-saver mode.
But when David Sinclair says that resveratrol or exercise or intermittent fasting or saunas act by “mimicking calorie restriction”, is he suggesting that they will make you weak and constantly tired? If not, why not? This sounds a denial of the fundamental mTOR tradeoff: less energy expenditure in exchange for worse performance. The impression I get from Lifespan is that all of these things will both make you live longer and make you healthier. That doesn’t really make sense to me.
(although so far the empirical evidence agrees with Sinclair and disagrees with my common sense, so probably I’m missing something.)
Finally, a friend wasn’t impressed with Sinclair’s clone argument. They point out: suppose aging is DNA damage, and it happens to every tenth cell. Having a tenth of your cells damaged is pretty bad, especially if they become senescent. “Senescent cells”, common in elderly people, have sustained so much damage that they can’t even die properly, and just sort of sit around being hopelessly confused and secreting random chemicals which freak out all the other cells around them. Everyone agrees these are an important part of aging, even if they’re not sure about the specifics. But if 1/10 of your cells are like this, then you have a 90% chance of grabbing a healthy cell for cloning. And even if you get a bad cell, no cloning process works every time, so you’ll just shrug and try again.
My impression of the consensus in anti-aging research is that many people are excited for the same reasons Sinclair is excited, that people are much more optimistic than they were five or ten years ago - but that their level of optimism hasn’t quite caught up to Sinclair’s level yet.
III.
Interspersed with all this stuff about mTOR and sirtuins is discussion of a broader question: is stopping aging desirable?
Sinclair thinks self-evidently yes. He tells the story of his grandmother - a Hungarian Jew who fled to Australia to escape communist oppression. She was adventurous, “young at heart”, and “she did her damnedest to live life with the spirit and awe of a child”. Sinclair remembers her as a happy person and free spirit who was always there for him and his family during their childhood in the Australian outback.
And her death was a drawn-out torture:
By her mid-80s, Vera was a shell of her former self, and the final decade of her life was hard to watch…Toward the end, she gave up hope. ‘This is just the way it goes’, she told me. She died at the age of 92…but the more I have thought about it, the more I have come to believe that the person she truly was had been dead many years at that point.
Sinclair’s mother didn’t have an easy time either:
It was a quick death, thankfully, caused by a buildup of liquid in her remaining lung. We had just been laughing together about the eulogy I’d written on the trip from the United States to Australia, and then suddenly she was writhing on the bed, sucking for air that couldn’t satisfy her body’s demand for oxygen, staring at us with desperation in her eyes.
I leaned in and whispered into her ear that she was the best mom I could have wished for. Within a few minutes, her neurons were dying, erasing not just the memory of my final words to her but all of her memories. I know some people die peacefully. But that’s not what happened to my mother. In those moments she was transformed from the person who had raised me into a twitching, choking mass of cells, all fighting over the last residues of energy being created at the atomic level of her being.
All I could think was “No one ever tells you what it is like to die. Why doesn’t anyone tell you?
It would be facile to say “and that’s what made him become an anti-aging researcher”. He was already an anti-aging researcher at that point. And more important, everyone has this experience. If seeing your loved ones fade into shells of their former selves and then die painfully reliably turned you into an anti-aging researcher, who would be left to do anything else?
So his first argument is something like “maybe the thing where we’re all forced to watch helplessly as the people we love the most all die painfully is bad, and we should figure out some solution”. It’s a pretty compelling argument, one which has inspired generations of alchemists, mystics, and spiritual seekers.
 An unexpectedly lovely kabbalistic correspondence on Lifespan’s cover. Sinclair’s name occupies the position of Yesod, the sephirah that bestows divine attributes onto the material world.
An unexpectedly lovely kabbalistic correspondence on Lifespan’s cover. Sinclair’s name occupies the position of Yesod, the sephirah that bestows divine attributes onto the material world.
But his second argument is: we put a lot of time and money into researching cures for cancer, heart disease, stroke, Alzheimers’, et cetera. Progress in these areas is bought dearly: all the low-hanging fruit has been picked, and what’s remaining is a grab bag of different complicated things - lung cancer is different from colon cancer is different from bone cancer.
The easiest way to cure cancer, Sinclair says, is to cure aging. Cancer risk per year in your 20s is only 1% what it is in your 80s. Keep everyone’s cells as healthy as they are in a 20-year-old, and you’ll cut cancer 99%, which is so close to a cure it hardly seems worth haggling over the remainder. As a bonus, you’ll get similar reductions in heart disease, stroke, Alzheimers, et cetera.
But also - to rehash the quote I started the review with - Sinclair thinks curing aging is easier than curing cancer. For one thing, aging might be just one thing, whereas cancer has lots of different types that need different strategies. For another, total cancer research spending approaches the hundreds of billions of dollars, whereas total anti-aging spending is maybe 0.1% of that. There’s a lot more low-hanging fruit!
And also, even if we succeed at curing cancer, it will barely matter on a population level. If we came up with a 100% perfect cure for cancer, average US life expectancy would increase two years - from 80 to 82. Add in a 100% perfect cure for heart disease, and you get 83. People mostly get these diseases when they are old, and old people are always going to die of something. Cure aging , and the whole concept of life expectancy goes out the window.
There are a lot of people who get angry about curing aging, because maybe God didn’t mean for us to be immortal, or maybe immortal billionaires will hog all the resources, or [insert lots of other things here]. One unambitious - but still potentially true - counterargument to this is that a world where we conquered aging, then euthanized everyone when they hit 80, would still be infinitely better than the current world where we age to 80 the normal way.
But once you’ve accepted this argument, there are some additional reasons to think conquering death would be good.
First, the environmental sustainability objection isn’t really that strong. If 50% of people stopped dying (maybe some people refuse the treatment, or can’t afford it), that would increase the US population by a little over a million people a year over the counterfactual where people die at the normal rate. That’s close to the annual number of immigrants. If you’re not worried about the sustainability of immigration, you probably shouldn’t worry about the sustainability of ending death.
You can make a similar argument for the world at large: life expectancy is a really minimal driver of population growth. The world’s longest-lived large country, Japan, currently has negative population growth; the world’s shortest-lived large country, Somalia, has one of the highest population growth rates in the world. If 25% of the world population took immortality serum (I’m decreasing this from the 50% for USA because I’m not even sure 50% of the world’s population has access to basic antibiotics), that would increase world population by 15 million per year over the counterfactual. It would take 60 years for there to even be an extra billion people, and in 60 years a lot of projections suggest world population will be stable or declining anyway. By the time we really have to worry about this we’ll either be dead or colonizing space.
Second, life expectancy at age 10 (ie excluding infant mortality) went up from about 45 in medieval Europe to about 85 in modern Europe. What bad things happened because of this? Modern Europe is currently in crisis because it has too few people and has to import immigrants from elsewhere in the world. And the increase didn’t cause some kind of stagnation where older people prevented society from ever changing. It didn’t cause some sort of perma-dictatorship where old people refuse to let go of their resources and the young toil for scraps. It corresponded to the period of the most rapid social and economic progress anywhere in history.
Would Europe be better off if the government killed every European the day they turned 45? If not, it seems like the experiment with extending life expectancy from 45 to 85 went pretty well. Why not try the experiment of extending life expectancy from 85 to 125, and see if that goes well too?
And finally, what’s the worst that could happen? An overly literal friend has a habit of always answering that question with “everyone in the world dies horribly”. But in this case, that’s what happens if we don’t do it. Seems like we have nowhere to go but up!